Online courses directory (423)
This seminar will focus on three sports: swimming, cycling and running. There will be two components to the seminar: classroom sessions and a "laboratory" in the form of a structured training program. The classroom component will introduce the students to the chemistry of their own biological system. With swimming, running and cycling as sample sports, students are encouraged to apply their knowledge to complete a triathlon shortly after the term.
The seminar is designed to look at the science of triathlons and sports from a molecular/chemical biological point of view. We will be able to use our own bodies to see how exercise affects the system, through observations written in a training journal. We will also improve the overall fitness of the class through maintaining a physical fitness program over the course of the term. The end of the term will have us all participate in a mini-triathlon.
Discover a world built by atoms, shaped by molecules and kept running with chemical reactions!
Acid Base Introduction. pH, pOH of Strong Acids and Bases. pH of a Weak Acid. pH of a Weak Base. Conjugate Acids and Bases. pKa and pKb Relationship. Buffers and Hendersen-Hasselbalch. Strong Acid Titration. Weak Acid Titration. Half Equivalence Point. Titration Roundup. Acid Base Titration. Acid Base Introduction. pH, pOH of Strong Acids and Bases. pH of a Weak Acid. pH of a Weak Base. Conjugate Acids and Bases. pKa and pKb Relationship. Buffers and Hendersen-Hasselbalch. Strong Acid Titration. Weak Acid Titration. Half Equivalence Point. Titration Roundup. Acid Base Titration.
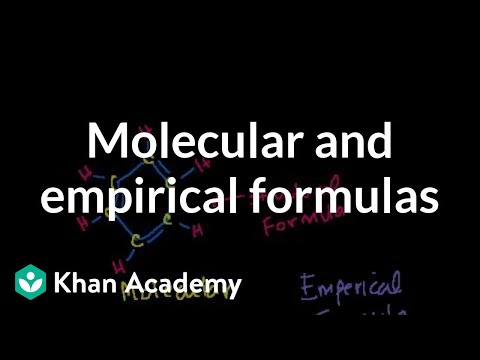
Molecular and Empirical Formulas. The Mole and Avogadro's Number. Formula from Mass Composition. Another mass composition problem. Balancing Chemical Equations. Stoichiometry. Stoichiometry Example Problem 1. Stoichiometry Example Problem 2. Stoichiometry: Limiting Reagent. Limiting Reactant Example Problem 1. Spectrophotometry Introduction. Spectrophotometry Example. Molecular and Empirical Formulas. The Mole and Avogadro's Number. Formula from Mass Composition. Another mass composition problem. Balancing Chemical Equations. Stoichiometry. Stoichiometry Example Problem 1. Stoichiometry Example Problem 2. Stoichiometry: Limiting Reagent. Limiting Reactant Example Problem 1. Spectrophotometry Introduction. Spectrophotometry Example.
This introduction to fundamental chemical concepts of atomic and molecular structure will emphasize the development of these concepts from experimental observations and scientific reasoning.
This course is the second semester of the two semester sequence, Chemistry Concept Development and Application. This course will cover the topics of a typical second semester General Chemistry course at most colleges and universities. We will use the Chemistry Concept Development Study approach, developed and used in our courses at Rice and used in Part I of this course.
Build your earth science vocabulary and learn about cycles of matter and types of sedimentary rocks through the Education Portal course Earth Science 101: Earth Science. Our series of video lessons and accompanying self-assessment quizzes can help you boost your scientific knowledge ahead of the Excelsior Earth Science exam . This course was designed by experienced educators and examines both science basics, like experimental design and systems of measurement, and more advanced topics, such as analysis of rock deformation and theories of continental drift.
Ideal Gas Equation: PV=nRT. Ideal Gas Equation Example 1. Ideal Gas Equation Example 2. Ideal Gas Equation Example 3. Ideal Gas Equation Example 4. Partial Pressure. Vapor Pressure Example. Ideal Gas Equation: PV=nRT. Ideal Gas Equation Example 1. Ideal Gas Equation Example 2. Ideal Gas Equation Example 3. Ideal Gas Equation Example 4. Partial Pressure. Vapor Pressure Example.
Elements and Atoms. Introduction to the atom. Elements and Atoms. Introduction to the atom.
Orbitals. More on orbitals and electron configuration. Electron configurations. Electron configurations 2. Valence Electrons. Orbitals. More on orbitals and electron configuration. Electron configurations. Electron configurations 2. Valence Electrons.
Introduction to Oxidation States. More on Oxidation States. Hydrogen Peroxide Correction. Redox Reactions. Galvanic Cells. Introduction to Oxidation States. More on Oxidation States. Hydrogen Peroxide Correction. Redox Reactions. Galvanic Cells.
Groups of the Periodic Table. Valence Electrons. Periodic Table Trends: Ionization Energy. Other Periodic Table Trends. Ionic, Covalent, and Metallic Bonds. Groups of the Periodic Table. Valence Electrons. Periodic Table Trends: Ionization Energy. Other Periodic Table Trends. Ionic, Covalent, and Metallic Bonds.
Types of Decay. Half-Life. Exponential Decay Formula Proof (can skip, involves Calculus). Introduction to Exponential Decay. More Exponential Decay Examples. Types of Decay. Half-Life. Exponential Decay Formula Proof (can skip, involves Calculus). Introduction to Exponential Decay. More Exponential Decay Examples.
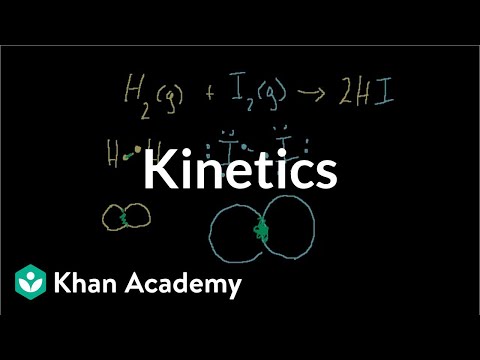
Introduction to Kinetics. Reactions in Equilibrium. Mini-Video on Ion Size. Keq Intuition (mathy and not necessary to progress). Keq derivation intuition (can skip; bit mathy). Heterogeneous Equilibrium. Le Chatelier's Principle. Introduction to pH, pOH, and pKw. Introduction to Kinetics. Reactions in Equilibrium. Mini-Video on Ion Size. Keq Intuition (mathy and not necessary to progress). Keq derivation intuition (can skip; bit mathy). Heterogeneous Equilibrium. Le Chatelier's Principle. Introduction to pH, pOH, and pKw.
States of Matter. States of Matter Follow-Up. Specific Heat, Heat of Fusion and Vaporization. Chilling Water Problem. Phase Diagrams. Van Der Waals Forces. Covalent Networks, Metallic, and Ionic Crystals. Vapor Pressure. Suspensions, Colloids and Solutions. Solubility. Boiling Point Elevation and Freezing Point Suppression. Change of State Example. States of Matter. States of Matter Follow-Up. Specific Heat, Heat of Fusion and Vaporization. Chilling Water Problem. Phase Diagrams. Van Der Waals Forces. Covalent Networks, Metallic, and Ionic Crystals. Vapor Pressure. Suspensions, Colloids and Solutions. Solubility. Boiling Point Elevation and Freezing Point Suppression. Change of State Example.
This course explains the general principles of chicken behaviour and welfare, and the behavioural and physiological indicators that can be used to assess welfare in chickens kept in hobby flocks through to commercial farms.
This course develops an interdisciplinary understanding of the social, political, economic and scientific perspectives on climate change.
Find out how climate change will affect us, why we should care about it, and what solutions we can employ.
This course views climate change from a variety of perspectives at the intersection of the natural sciences, technology, and the social sciences and humanities.
Trusted paper writing service WriteMyPaper.Today will write the papers of any difficulty.