Online courses directory (423)
Lectures and discussions in this course cover the clinical, behavioral, and molecular aspects of the brain aging processes in humans. Topics include the loss of memory and other cognitive abilities in normal aging, as well as neurodegenerative conditions such as Parkinson's and Alzheimer's diseases. Discussions based on readings taken from primary literature explore the current research in this field.
The neuropharmacology course will discuss the drug-induced changes in functioning of the nervous system. The specific focus of this course will be to provide a description of the cellular and molecular actions of drugs on synaptic transmission. This course will also refer to specific diseases of the nervous system and their treatment in addition to giving an overview of the techniques used for the study of neuropharmacology.
This course is offered during the Independent Activities Period (IAP), which is a special 4-week term at MIT that runs from the first week of January until the end of the month.
Numerical methods for solving problems arising in heat and mass transfer, fluid mechanics, chemical reaction engineering, and molecular simulation. Topics: numerical linear algebra, solution of nonlinear algebraic equations and ordinary differential equations, solution of partial differential equations (e.g. Navier-Stokes), numerical methods in molecular simulation (dynamics, geometry optimization). All methods are presented within the context of chemical engineering problems. Familiarity with structured programming is assumed. The examples will use MATLAB®.
Acknowledgements
The instructor would like to thank Robert Ashcraft, Sandeep Sharma, David Weingeist, and Nikolay Zaborenko for their work in preparing materials for this course site.
This seven week course will explore nutrition concepts that take center stage in mainstream media outlets and become conversation topics among consumers interested in food choice as it relates to optimal health and physical performance.
Provides a comprehensive introduction to key issues and findings in object recognition in experimental, neural, computational, and applied domains. Emphasizes the problem of representation, exploring the issue of how 3-D objects should be encoded so as to efficiently recognize them from 2-D images. Second half focuses on face recognition, an ecologically important instance of the general object recognition problem. Describes experimental studies of human face recognition performance and recent attempts to mimic this ability in artificial computational systems.
This course will look at how intelligent and innovative use of the ocean can sustainably deliver the key resources necessary to help meet some of the great challenges faced by humanity.
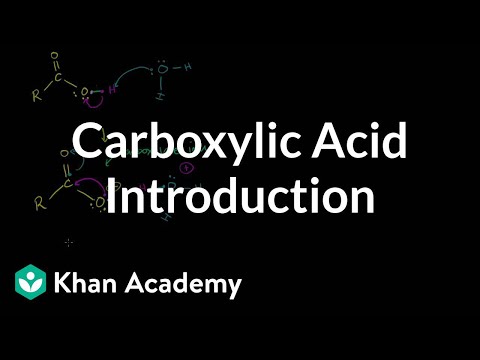
Topics covered in college organic chemistry course. Basic understanding of basic high school or college chemistry assumed. Representing Structures of Organic Molecules. Naming Simple Alkanes. Naming Alkanes with Alkyl Groups. Correction - 2-Propylheptane should never be the name!. Common and Systematic Naming-Iso, Sec and Tert Prefixes. Organic Chemistry Naming Examples 1. Organic Chemistry Naming Examples 2. Organic Chemistry Naming Examples 3. Organic Chemistry Naming Examples 4. Organic Chemistry Naming Examples 5. Naming Alkenes Examples. Naming Alkyl Halides. sp3 Hybridized Orbitals and Sigma Bonds. Pi bonds and sp2 Hybridized Orbitals. Newman Projections. Newman Projections 2. Chair and Boat Shapes for Cyclohexane. Double Newman Diagram for Methcyclohexane. Introduction to Chirality. Chiral Examples 1. Chiral Examples 2. Cahn-Ingold-Prelog System for Naming Enantiomers. R,S (Cahn-Ingold-Prelog) Naming System Example 2. Stereoisomers, Enantiomers, Diastereomers, Constitutional Isomers and Meso Compounds. Cis-Trans and E-Z Naming Scheme for Alkenes. Entgegen-Zusammen Naming Scheme for Alkenes Examples. Introduction to Reaction Mechanisms. Markovnikov's Rule and Carbocations. Addition of Water (Acid-Catalyzed) Mechanism. Polymerization of Alkenes with Acid. Sn2 Reactions. Sn1 Reactions. Steric Hindrance. Sn2 Stereochemistry. Solvent Effects on Sn1 and Sn2 Reactions. Nucleophilicity (Nucleophile Strength). Nucleophilicity vs. Basicity. E2 Reactions. E1 Reactions. Zaitsev's Rule. Comparing E2 E1 Sn2 Sn1 Reactions. E2 E1 Sn2 Sn1 Reactions Example 2. E2 E1 Sn2 Sn1 Reactions Example 3. Free Radical Reactions. Alcohols. Alcohol Properties. Resonance. Ether Naming and Introduction. Cyclic ethers and epoxide naming. Ring-opening Sn2 reaction of expoxides. Sn1 and Sn2 epoxide opening discussion. Aromatic Compounds and Huckel's Rule. Naming Benzene Derivatives Introduction. Electrophilic Aromatic Substitution. Bromination of Benzene. Amine Naming Introduction. Amine Naming 2. Amine as Nucleophile in Sn2 Reaction. Amine in Sn2 part 2. Sn1 Amine Reaction. Aldehyde Introduction. Ketone Naming. Friedel Crafts Acylation. Friedel Crafts Acylation Addendum. Keto Enol Tautomerization. Carboxlic Acid Introduction. Carboxylic Acid Naming. Fisher Esterification. Acid Chloride Formation. Amides, Anhydrides, Esters and Acyl Chlorides. Relative Stability of Amides Esters Anhydrides and Acyl Chlorides. Amide Formation from Acyl Chloride. Aldol Reaction.
alcohols, ethers, epoxides, thiols, sulfides. Alcohols. Alcohol Properties. alcohol nomenclature. physical properties of alcohols and preparation of alkoxides. preparation of alcohols using NaBH4. preparation of alcohols using LiAlH4. synthesis of alcohols using grignard reagents I. synthesis of alcohols using grignard reagents II. oxidation of alcohols I: mechanism and oxidation states. oxidation of alcohols II: examples. biological redox reactions. formation of nitrate esters. preparation of alkyl halides from alcohols. Ether Naming and Introduction. Ether nomenclature. Properties of ethers and crown ethers. williamson ether synthesis. acidic cleavage of ethers. Cyclic ethers and epoxide naming. nomenclature and preparation of epoxides. preparation of epoxides: stereochemistry. Ring-opening Sn2 reaction of expoxides. Sn1 and Sn2 epoxide opening discussion. ring-opening reactions of epoxides: strong nucleophiles. ring opening reactions of epoxides: acid-catalyzed. preparation of sulfides. Alcohols. Alcohol Properties. alcohol nomenclature. physical properties of alcohols and preparation of alkoxides. preparation of alcohols using NaBH4. preparation of alcohols using LiAlH4. synthesis of alcohols using grignard reagents I. synthesis of alcohols using grignard reagents II. oxidation of alcohols I: mechanism and oxidation states. oxidation of alcohols II: examples. biological redox reactions. formation of nitrate esters. preparation of alkyl halides from alcohols. Ether Naming and Introduction. Ether nomenclature. Properties of ethers and crown ethers. williamson ether synthesis. acidic cleavage of ethers. Cyclic ethers and epoxide naming. nomenclature and preparation of epoxides. preparation of epoxides: stereochemistry. Ring-opening Sn2 reaction of expoxides. Sn1 and Sn2 epoxide opening discussion. ring-opening reactions of epoxides: strong nucleophiles. ring opening reactions of epoxides: acid-catalyzed. preparation of sulfides.
nomenclature and reactions of aldehydes and ketones. Aldehyde Introduction. Ketone Naming. Keto Enol Tautomerization. Aldol Reaction. Aldehyde Introduction. Ketone Naming. Keto Enol Tautomerization. Aldol Reaction.
naming alkanes and cycloalkanes, conformations of alkanes and cycloalkanes, and free radical reactions. Representing Structures of Organic Molecules. Naming Simple Alkanes. Naming Alkanes with Alkyl Groups. Correction - 2-Propylheptane should never be the name!. Common and Systematic Naming-Iso, Sec and Tert Prefixes. Organic Chemistry Naming Examples 1. Organic Chemistry Naming Examples 2. Organic Chemistry Naming Examples 3. Organic Chemistry Naming Examples 4. Organic Chemistry Naming Examples 5. Newman Projections. Newman Projections 2. Chair and Boat Shapes for Cyclohexane. Double Newman Diagram for Methcyclohexane. alkane and cycloalkane nomenclature I. alkane and cycloalkane nomenclature II. alkane and cycloalkane nomenclature III. bicyclic compounds. naming cubane. conformations of ethane and propane. conformations of butane. conformations of cyclohexane I: chair and boat. conformations of cyclohexane II: monosubstituted. conformations of cyclohexane III: disubstituted. conformations of cyclohexane IV: trisubstituted. Free Radical Reactions. Representing Structures of Organic Molecules. Naming Simple Alkanes. Naming Alkanes with Alkyl Groups. Correction - 2-Propylheptane should never be the name!. Common and Systematic Naming-Iso, Sec and Tert Prefixes. Organic Chemistry Naming Examples 1. Organic Chemistry Naming Examples 2. Organic Chemistry Naming Examples 3. Organic Chemistry Naming Examples 4. Organic Chemistry Naming Examples 5. Newman Projections. Newman Projections 2. Chair and Boat Shapes for Cyclohexane. Double Newman Diagram for Methcyclohexane. alkane and cycloalkane nomenclature I. alkane and cycloalkane nomenclature II. alkane and cycloalkane nomenclature III. bicyclic compounds. naming cubane. conformations of ethane and propane. conformations of butane. conformations of cyclohexane I: chair and boat. conformations of cyclohexane II: monosubstituted. conformations of cyclohexane III: disubstituted. conformations of cyclohexane IV: trisubstituted. Free Radical Reactions.
naming alkenes and alkynes, reactions of alkenes and alkynes, synthesis. Naming Alkenes Examples. Cis-Trans and E-Z Naming Scheme for Alkenes. Entgegen-Zusammen Naming Scheme for Alkenes Examples. Introduction to Reaction Mechanisms. Markovnikov's Rule and Carbocations. Addition of Water (Acid-Catalyzed) Mechanism. Polymerization of Alkenes with Acid. Alkene intro and stability. Alkene nomenclature. cis/trans and the E/Z system. hydrogenation. hydrohalogenation. hydration. halogenation. halohydrin formation. hydroboration-oxidation. epoxide formation and anti dihydroxylation. syn dihydroxylation. Ozonolysis. alkyne nomenclature. alkyne acidity and alkylation. preparation of alkynes. reduction of alkynes. hydrohalogenation of alkynes. hydration of alkynes. hydroboration-oxidation of alkynes. halogenation and ozonolysis of alkynes. synthesis using alkynes. Naming Alkenes Examples. Cis-Trans and E-Z Naming Scheme for Alkenes. Entgegen-Zusammen Naming Scheme for Alkenes Examples. Introduction to Reaction Mechanisms. Markovnikov's Rule and Carbocations. Addition of Water (Acid-Catalyzed) Mechanism. Polymerization of Alkenes with Acid. Alkene intro and stability. Alkene nomenclature. cis/trans and the E/Z system. hydrogenation. hydrohalogenation. hydration. halogenation. halohydrin formation. hydroboration-oxidation. epoxide formation and anti dihydroxylation. syn dihydroxylation. Ozonolysis. alkyne nomenclature. alkyne acidity and alkylation. preparation of alkynes. reduction of alkynes. hydrohalogenation of alkynes. hydration of alkynes. hydroboration-oxidation of alkynes. halogenation and ozonolysis of alkynes. synthesis using alkynes.
nomenclature and reactions of amines. Amine Naming Introduction. Amine Naming 2. Amine as Nucleophile in Sn2 Reaction. Amine in Sn2 part 2. Sn1 Amine Reaction. Amine Naming Introduction. Amine Naming 2. Amine as Nucleophile in Sn2 Reaction. Amine in Sn2 part 2. Sn1 Amine Reaction.
aromatic compounds, naming derivatives of benzene, electrophilic aromatic substitution reactions. Naming Benzene Derivatives Introduction. naming benzene derivatives. Aromatic Compounds and Huckel's Rule. Aromatic Stability I. aromatic stability II. aromatic stability III. aromatic stability IV. aromatic stability V. Aromatic Heterocycles I. Aromatic Heterocycles II. Resonance. Electrophilic Aromatic Substitution. Bromination of Benzene. Friedel Crafts Acylation. Friedel Crafts Acylation Addendum. Electrophilic Aromatic Substitution Mechanism. Halogenation. Nitration. Sulfonation. Friedel-Crafts Alkylation. Friedel-Crafts Acylation. Ortho-Para Directors I. Ortho-Para Directors II. Ortho-Para Directors III. Meta Directors I. Meta Directors II. Multiple Substituents. Birch Reduction I. Birch Reduction II. Reactions at the Benzylic Position. Synthesis of Substituted Benzene Rings I. Synthesis of Substituted Benzene Rings II. Nucleophilic Aromatic Substitution I. Nucleophilic Aromatic Substitution II. Naming Benzene Derivatives Introduction. naming benzene derivatives. Aromatic Compounds and Huckel's Rule. Aromatic Stability I. aromatic stability II. aromatic stability III. aromatic stability IV. aromatic stability V. Aromatic Heterocycles I. Aromatic Heterocycles II. Resonance. Electrophilic Aromatic Substitution. Bromination of Benzene. Friedel Crafts Acylation. Friedel Crafts Acylation Addendum. Electrophilic Aromatic Substitution Mechanism. Halogenation. Nitration. Sulfonation. Friedel-Crafts Alkylation. Friedel-Crafts Acylation. Ortho-Para Directors I. Ortho-Para Directors II. Ortho-Para Directors III. Meta Directors I. Meta Directors II. Multiple Substituents. Birch Reduction I. Birch Reduction II. Reactions at the Benzylic Position. Synthesis of Substituted Benzene Rings I. Synthesis of Substituted Benzene Rings II. Nucleophilic Aromatic Substitution I. Nucleophilic Aromatic Substitution II.
naming carboxylic acids, formation of carboxylic acid derivatives. Carboxlic Acid Introduction. Carboxylic Acid Naming. Fisher Esterification. Acid Chloride Formation. Amides, Anhydrides, Esters and Acyl Chlorides. Relative Stability of Amides Esters Anhydrides and Acyl Chlorides. Amide Formation from Acyl Chloride. Carboxlic Acid Introduction. Carboxylic Acid Naming. Fisher Esterification. Acid Chloride Formation. Amides, Anhydrides, Esters and Acyl Chlorides. Relative Stability of Amides Esters Anhydrides and Acyl Chlorides. Amide Formation from Acyl Chloride.
conjugation, conjugated dienes, addition reactions of conjugated dienes, diels-alder reaction, MO theory, color. addition reaction of conjugated dienes I: mechanism. addition reaction of conjugated dienes II: example. addition reaction of conjugated dienes III: control. diels-alder I: mechanism. diels-alder II: endo vs exo. diels-alder III: stereochemistry of dienophile. diels-alder IV: stereochemistry of diene. diels-alder V: regiochemistry. diels-alder VI: more regiochemistry. diels-alder VII: intramolecular. intro to molecular orbital (MO) theory. MO theory for butadiene. MO theory for Diels-Alder. intro to color theory. conjugation and color. color in organic molecules. addition reaction of conjugated dienes I: mechanism. addition reaction of conjugated dienes II: example. addition reaction of conjugated dienes III: control. diels-alder I: mechanism. diels-alder II: endo vs exo. diels-alder III: stereochemistry of dienophile. diels-alder IV: stereochemistry of diene. diels-alder V: regiochemistry. diels-alder VI: more regiochemistry. diels-alder VII: intramolecular. intro to molecular orbital (MO) theory. MO theory for butadiene. MO theory for Diels-Alder. intro to color theory. conjugation and color. color in organic molecules.
A review of hybrid orbitals, dot structures, electronegativity, and polarity. sp3 Hybridized Orbitals and Sigma Bonds. Pi bonds and sp2 Hybridized Orbitals. dot structures I: single bonds. dot structures II: multiple bonds. sp3 hybrid orbitals. tetrahedral bond angle proof. sp2 hybrid orbitals. sp hybrid orbitals. more hybridization. electronegativity. electronegativity and intermolecular forces. sp3 Hybridized Orbitals and Sigma Bonds. Pi bonds and sp2 Hybridized Orbitals. dot structures I: single bonds. dot structures II: multiple bonds. sp3 hybrid orbitals. tetrahedral bond angle proof. sp2 hybrid orbitals. sp hybrid orbitals. more hybridization. electronegativity. electronegativity and intermolecular forces.
bond-line structures, functional groups, formal charges, resonance structures, oxidation and reduction, acid/base chemistry. bond-line structures. 3-D bond-line structures. structural (constitutional) isomers. functional groups I. functional groups II. formal charge I. formal charge II. resonance structures I. resonance structures II. resonance structures III. oxidation states I. oxidation states II. Acid/Base Definitions. Ka and pKa Derivation. Stabilization of Conjugate Base I. Stabilization of Conjugate Base II. Stabilization of Conjugate Base III. Stabilization of Conjugate Base IV. bond-line structures. 3-D bond-line structures. structural (constitutional) isomers. functional groups I. functional groups II. formal charge I. formal charge II. resonance structures I. resonance structures II. resonance structures III. oxidation states I. oxidation states II. Acid/Base Definitions. Ka and pKa Derivation. Stabilization of Conjugate Base I. Stabilization of Conjugate Base II. Stabilization of Conjugate Base III. Stabilization of Conjugate Base IV.
chirality, stereoisomers, assigning absolute configuration using the R,S system, optical activity, diastereomers, meso compounds, fischer projections. Introduction to Chirality. Chiral Examples 1. Chiral Examples 2. Cahn-Ingold-Prelog System for Naming Enantiomers. R,S (Cahn-Ingold-Prelog) Naming System Example 2. chirality centers and stereoisomers. R,S system for determining absolute configuration. R,S system for cyclic compounds. optical activity I: theory. optical activity II: calculations. Stereoisomers, Enantiomers, Diastereomers, Constitutional Isomers and Meso Compounds. diastereomers. meso compounds. fischer projections. Introduction to Chirality. Chiral Examples 1. Chiral Examples 2. Cahn-Ingold-Prelog System for Naming Enantiomers. R,S (Cahn-Ingold-Prelog) Naming System Example 2. chirality centers and stereoisomers. R,S system for determining absolute configuration. R,S system for cyclic compounds. optical activity I: theory. optical activity II: calculations. Stereoisomers, Enantiomers, Diastereomers, Constitutional Isomers and Meso Compounds. diastereomers. meso compounds. fischer projections.
SN1, SN2, E1, E2, nucleophiles, nucleophilicity, basicity. Naming Alkyl Halides. Sn2 Reactions. Sn1 Reactions. Steric hindrance. Sn2 Stereochemistry. Solvent Effects on Sn1 and Sn2 Reactions. Nucleophilicity (Nucleophile Strength). Nucleophilicity vs. Basicity. E2 Reactions. E1 Reactions. Zaitsev's Rule. Comparing E2 E1 Sn2 Sn1 Reactions. E2 E1 Sn2 Sn1 Reactions Example 2. E2 E1 Sn2 Sn1 Reactions Example 3. nucleophile/electrophile and The Schwartz Rules. alkyl halide nomenclature. SN1 reaction: mechanism. SN1 reaction: stereochemistry. SN2 mechanism and stereochemistry. SN1 vs SN2: solvent effects. SN1 vs SN2: summary. E1 Elimination: mechanism. E1 Elimination: regioselectivity and stereoselectivity. carbocations and rearrangements. E1 Elimination: carbocation rearrangements. E2 Elimination: mechanism. E2 Elimination: regioselectivity. E2 Elimination: stereoselectivity. E2 Elimination: stereospecificity. E2 Elimination: substituted cyclohexanes. nucleophilicity and basicity. SN1 SN2 E1 E2 reactions: primary and tertiary alkyl halides. SN1 SN2 E1 E2 reactions: secondary alkyl halides. Naming Alkyl Halides. Sn2 Reactions. Sn1 Reactions. Steric hindrance. Sn2 Stereochemistry. Solvent Effects on Sn1 and Sn2 Reactions. Nucleophilicity (Nucleophile Strength). Nucleophilicity vs. Basicity. E2 Reactions. E1 Reactions. Zaitsev's Rule. Comparing E2 E1 Sn2 Sn1 Reactions. E2 E1 Sn2 Sn1 Reactions Example 2. E2 E1 Sn2 Sn1 Reactions Example 3. nucleophile/electrophile and The Schwartz Rules. alkyl halide nomenclature. SN1 reaction: mechanism. SN1 reaction: stereochemistry. SN2 mechanism and stereochemistry. SN1 vs SN2: solvent effects. SN1 vs SN2: summary. E1 Elimination: mechanism. E1 Elimination: regioselectivity and stereoselectivity. carbocations and rearrangements. E1 Elimination: carbocation rearrangements. E2 Elimination: mechanism. E2 Elimination: regioselectivity. E2 Elimination: stereoselectivity. E2 Elimination: stereospecificity. E2 Elimination: substituted cyclohexanes. nucleophilicity and basicity. SN1 SN2 E1 E2 reactions: primary and tertiary alkyl halides. SN1 SN2 E1 E2 reactions: secondary alkyl halides.
Develop a greater appreciation for how the air, water, land, and life formed and have interacted over the last 4.5 billion years.
Trusted paper writing service WriteMyPaper.Today will write the papers of any difficulty.