Courses tagged with "Diencephalon" (158)
This first-year University chemistry course explores the basic principles of the chemical bond by studying the properties of solids. Properties such as stiffness, electrical conductivity, thermal expansion, strength, and optical properties are the vehicle by which you can learn a great deal of practical chemistry.
You will see how experts use their knowledge of trends in the periodic table to predict the properties of materials. 3.091x is an engineering course so there is an emphasis on applications and how materials are used. The on-campus version of the course has been taught for over forty years and is one of the largest classes at MIT.
This course will cover the relationship between electronic structure, chemical bonding, and atomic order, and characterization of atomic arrangements in crystalline and amorphous solids: metals, ceramics, semiconductors, and polymers (including proteins). There will be topical coverage of organic chemistry, solution chemistry, acid-base equilibria, electrochemistry, biochemistry, chemical kinetics, diffusion, and phase diagrams. Examples will be drawn from industrial practice (including the environmental impact of chemical processes), from energy generation and storage (e.g., batteries and fuel cells), and from emerging technologies (e.g., photonic and biomedical devices).
Organic chemistry course surveying introductory topics in structure and reactivity with an emphasis on elementary reaction mechanisms.
Acid Base Introduction. pH, pOH of Strong Acids and Bases. pH of a Weak Acid. pH of a Weak Base. Conjugate Acids and Bases. pKa and pKb Relationship. Buffers and Hendersen-Hasselbalch. Strong Acid Titration. Weak Acid Titration. Half Equivalence Point. Titration Roundup. Acid Base Titration. Acid Base Introduction. pH, pOH of Strong Acids and Bases. pH of a Weak Acid. pH of a Weak Base. Conjugate Acids and Bases. pKa and pKb Relationship. Buffers and Hendersen-Hasselbalch. Strong Acid Titration. Weak Acid Titration. Half Equivalence Point. Titration Roundup. Acid Base Titration.
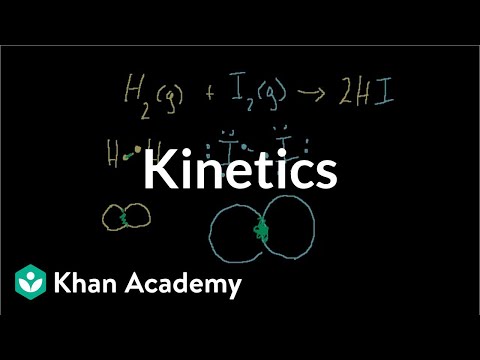
Introduction to Kinetics. Reactions in Equilibrium. Mini-Video on Ion Size. Keq Intuition (mathy and not necessary to progress). Keq derivation intuition (can skip; bit mathy). Heterogeneous Equilibrium. Le Chatelier's Principle. Introduction to pH, pOH, and pKw. Introduction to Kinetics. Reactions in Equilibrium. Mini-Video on Ion Size. Keq Intuition (mathy and not necessary to progress). Keq derivation intuition (can skip; bit mathy). Heterogeneous Equilibrium. Le Chatelier's Principle. Introduction to pH, pOH, and pKw.
This course will focus on the theory, design and operation of commercial nuclear power reactors. The course will also touch on contemporary issues regarding nuclear power generation including: the nuclear fuel cycle, the economics of nuclear power, and nuclear non-proliferation.
Types of Decay. Half-Life. Exponential Decay Formula Proof (can skip, involves Calculus). Introduction to Exponential Decay. More Exponential Decay Examples. Types of Decay. Half-Life. Exponential Decay Formula Proof (can skip, involves Calculus). Introduction to Exponential Decay. More Exponential Decay Examples.
This is a organic chemistry course surveying introductory topics in structure and reactivity with an emphasis on structural fundamentals including electronic structure, conformation and stereochemistry.
Elements and Atoms. Introduction to the atom. Elements and Atoms. Introduction to the atom.
States of Matter. States of Matter Follow-Up. Specific Heat, Heat of Fusion and Vaporization. Chilling Water Problem. Phase Diagrams. Van Der Waals Forces. Covalent Networks, Metallic, and Ionic Crystals. Vapor Pressure. Suspensions, Colloids and Solutions. Solubility. Boiling Point Elevation and Freezing Point Suppression. Change of State Example. States of Matter. States of Matter Follow-Up. Specific Heat, Heat of Fusion and Vaporization. Chilling Water Problem. Phase Diagrams. Van Der Waals Forces. Covalent Networks, Metallic, and Ionic Crystals. Vapor Pressure. Suspensions, Colloids and Solutions. Solubility. Boiling Point Elevation and Freezing Point Suppression. Change of State Example.
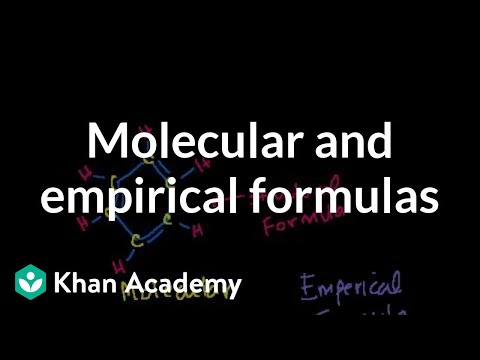
Molecular and Empirical Formulas. The Mole and Avogadro's Number. Formula from Mass Composition. Another mass composition problem. Balancing Chemical Equations. Stoichiometry. Stoichiometry Example Problem 1. Stoichiometry Example Problem 2. Stoichiometry: Limiting Reagent. Limiting Reactant Example Problem 1. Spectrophotometry Introduction. Spectrophotometry Example. Molecular and Empirical Formulas. The Mole and Avogadro's Number. Formula from Mass Composition. Another mass composition problem. Balancing Chemical Equations. Stoichiometry. Stoichiometry Example Problem 1. Stoichiometry Example Problem 2. Stoichiometry: Limiting Reagent. Limiting Reactant Example Problem 1. Spectrophotometry Introduction. Spectrophotometry Example.
Introduction to Oxidation States. More on Oxidation States. Hydrogen Peroxide Correction. Redox Reactions. Galvanic Cells. Introduction to Oxidation States. More on Oxidation States. Hydrogen Peroxide Correction. Redox Reactions. Galvanic Cells.
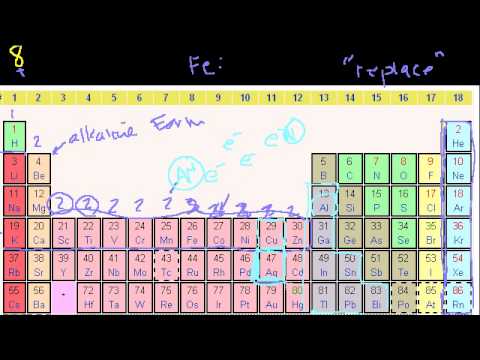
Orbitals. More on orbitals and electron configuration. Electron configurations. Electron configurations 2. Valence Electrons. Orbitals. More on orbitals and electron configuration. Electron configurations. Electron configurations 2. Valence Electrons.
Organic chemistry course covering intermediate topics in structure and reactivity with special applications to the life sciences.
Ideal Gas Equation: PV=nRT. Ideal Gas Equation Example 1. Ideal Gas Equation Example 2. Ideal Gas Equation Example 3. Ideal Gas Equation Example 4. Partial Pressure. Vapor Pressure Example. Ideal Gas Equation: PV=nRT. Ideal Gas Equation Example 1. Ideal Gas Equation Example 2. Ideal Gas Equation Example 3. Ideal Gas Equation Example 4. Partial Pressure. Vapor Pressure Example.
If chemistry is the science of stuff, then analytical chemistry answers the question: what is it? And how much of it do you have? This course teaches how to do this with instrumental analysis!
Organic chemistry course covering intermediate topics in structure and reactivity with emphasis on electronic structure, pericyclic reactions and aromatic heterocycles.
Nanotechnology is an emerging area that engages almost every technical discipline – from chemistry to computer science – in the study and application of extremely tiny materials. This short course allows any technically savvy person to go one layer beyond the surface of this broad topic to see the real substance behind the very small.
This introduction to fundamental chemical concepts of atomic and molecular structure will emphasize the development of these concepts from experimental observations and scientific reasoning.
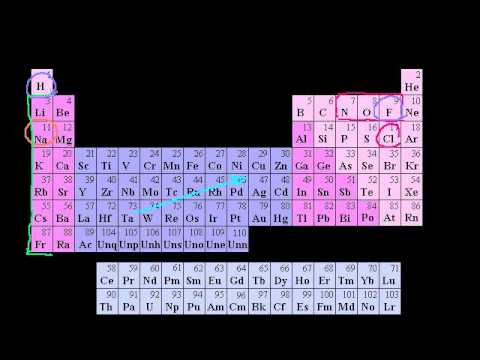
Groups of the Periodic Table. Valence Electrons. Periodic Table Trends: Ionization Energy. Other Periodic Table Trends. Ionic, Covalent, and Metallic Bonds. Groups of the Periodic Table. Valence Electrons. Periodic Table Trends: Ionization Energy. Other Periodic Table Trends. Ionic, Covalent, and Metallic Bonds.
Let’s make history together - again! 让我们再一次创造历史!
Trusted paper writing service WriteMyPaper.Today will write the papers of any difficulty.